Conserving sea turtles in a changing world
Sea turtle populations were drastically depleted from historical (pre-Columbian) levels due to human pressures including harvest, fisheries by-catch, and habitat destruction. While modern conservation efforts have made headway against some of these human threats, some persist (by-catch chief among them), and the modern era of global environmental change is posing a new suite of challenges.
As a member of the Marine Turtle Ecology & Assessment Program at NOAA’s Southwest Fisheries Science Center (La Jolla, California), as well as via my affiliations with the Blue Corridors for Turtles project and the Jumby Bay Hawksbill Project (Antigua, Eastern Caribbean), I seek to support sea turtle conservation and population recovery in the context of persistent human pressures and global change.
Spatial ecology & biotelemetry
Conservation, at a very fundamental level, depends on information showing where animals occur, what types of habitats they use, and how they move within said habitats. I use biotelemetry, GIS, and spatial modeling to generate this information.
Satellite telemetry —

To support the recovery of imperiled sea turtle populations, we need to know where turtles occur and how they move through managed waters. I use satellite telemetry to get at this information. For example, in the above picture I am using epoxy to attach a satellite transmitter to a nesting hawksbill with the help of Jumby Bay Hawksbill Project field researchers. We then tracked its post-reproductive migration to a foraging area. See below, or this map, this paper, or this paper, for examples of migrations from the Eastern Caribbean island of Antigua.
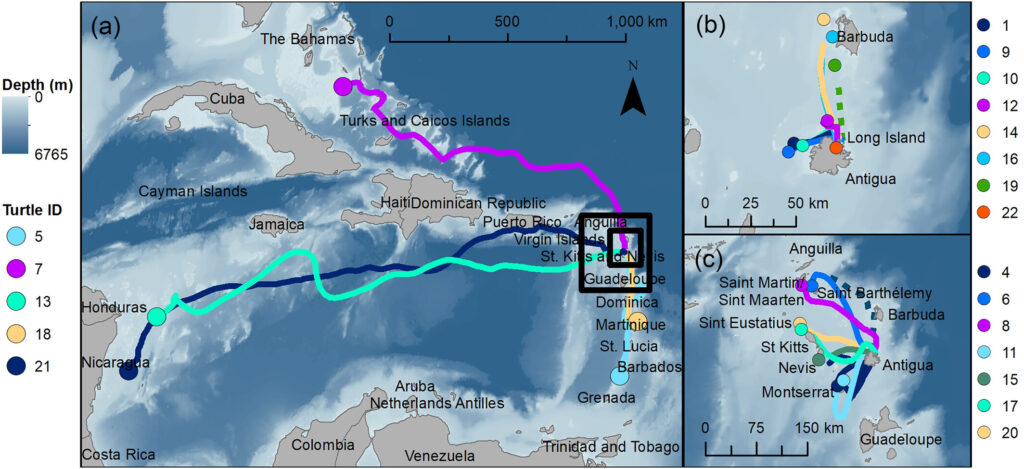
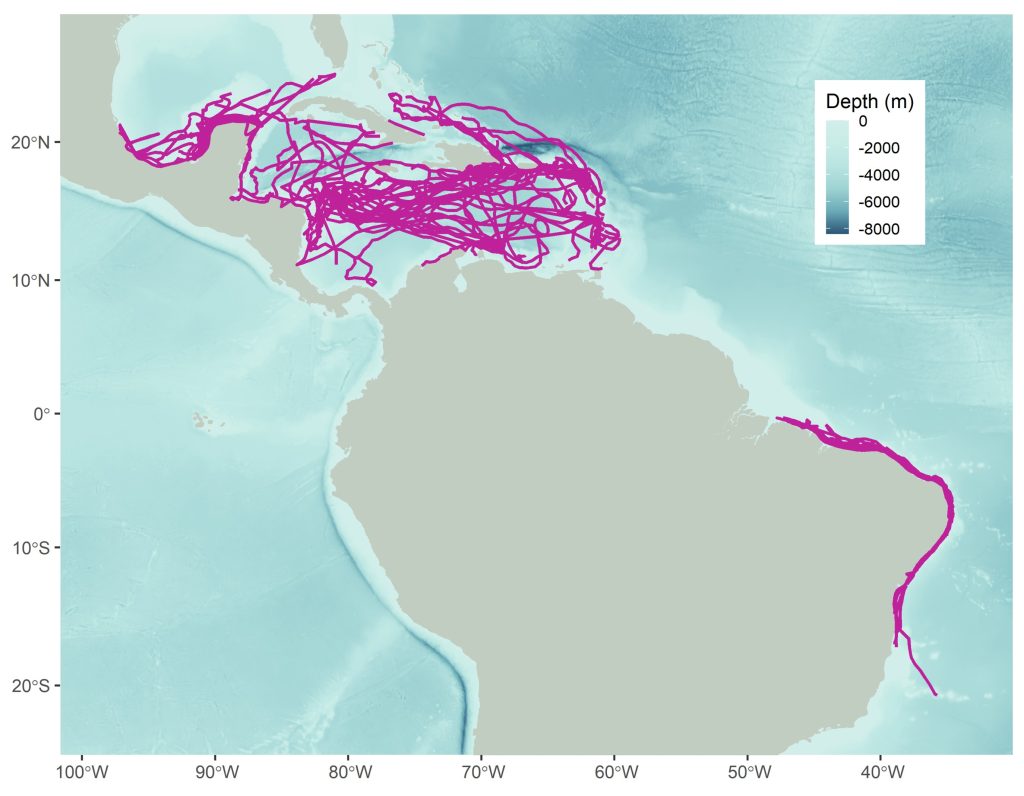
As my research has progressed over time, I have increasingly sought to understand distributions and migratory connectivity at larger scales. I work with some amazing colleagues to ask: rather than focusing on a single population, what can we learn by pooling available data for many populations? Some initial related findings for hawksbills in the Western Atlantic are depicted below and were published here. But what about patterns that apply across multiple species, and beyond just the Western Atlantic? Via new work with the Blue Corridors for Turtles project, that is where things are headed. Stay posted!
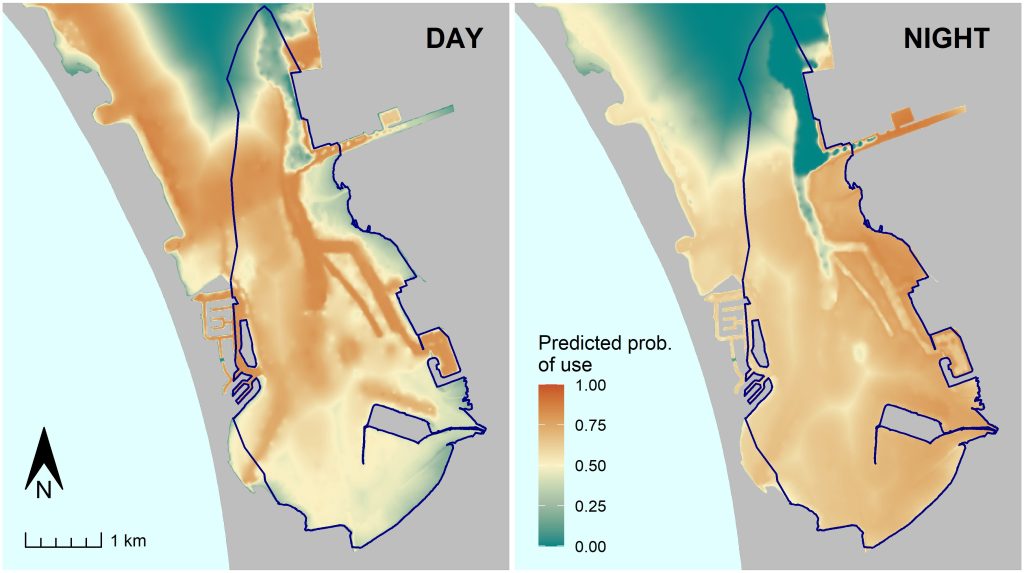
While large-scale migrations are fascinating, conservation efforts also require information on how individuals move at much finer scales. Many sea turtles spend most of their lives within foraging home ranges that can be quite small. At this finer scale, high-accuracy GPS data become very valuable. In my work with the Marine Turtle Ecology & Assessment Program at NOAA’s Southwest Fisheries Science Center, one of the many ways we have used GPS data is to document resource selection; that is, how turtles select for habitat features such as eelgrass and bathymetry (see our paper here). We can use resource selection model to make predictions of where turtles are most likely to occur depending on the size of the turtle, time of day, and season. See the map below for an example of such a prediction.
Animal-borne biologgers —
Beyond satellite transmitters, I use other animal-borne sensors to generate information on 3D movement and energy use. Below, my mentee and collaborator Aniela Anuszczyk and I are using suction cups to attach a multi-sensor pop-off camera to a green turtle in Southern California. This camera, manufactured by Customized Animal Tracking Solutions (CATS), allows us to observe what turtles eat and how they behave in their local environment, all while providing huge amounts of complementary sensor data (e.g., depth, temperature, accelerometry). We have occasionally deployed it alongside a satellite transmitter, as you can see in a second photo with mentee/collaborator Cameron Mullaney.


The CATS camera provides a unique, fine-scale look at the daily lives of California green turtles. One initial finding: green turtles in San Diego Bay are quite social! Cam Mullaney led an initial paper on the subject, culminating his senior thesis work at UCSD. (Research permitted under NOAA permit #18238).

Global change
Rising temperatures —
The rise in atmospheric temperatures has major implications for sea turtles that exhibit temperature-dependent sex determination (TSD), where warmer temperatures lead to the production of more female hatchlings. High temperatures can also reduce survival of incubating embryos. One wing of my research seeks to improve our understanding of how thermal effects on offspring will translate to changes in population dynamics. For example, I led a review on these subjects in the journal BioScience.
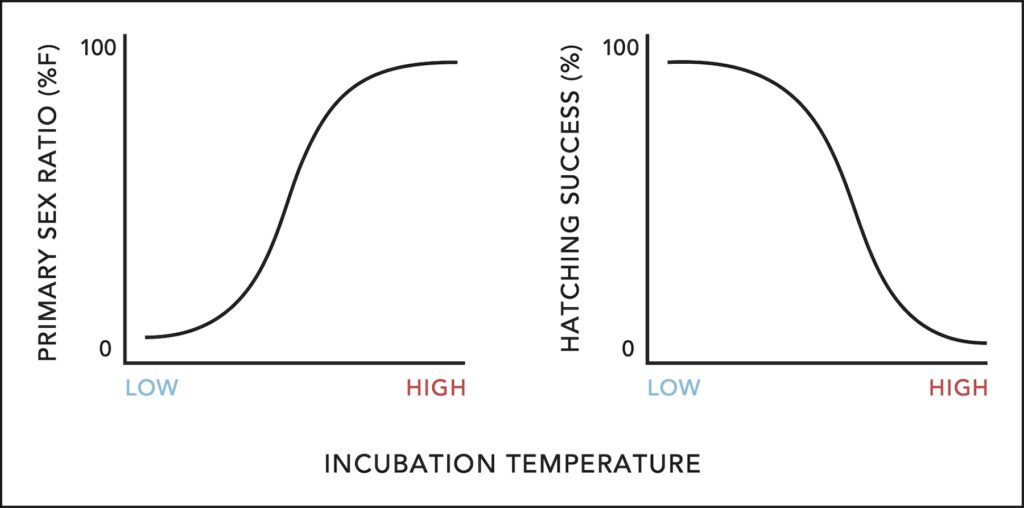
I have also led projects examining incubation temperatures in the field to understand the effects of warming temperatures on Antiguan hawksbills. The figure below shows temperatures in two nests (green and blue lines) monitored with data loggers in 2015. We can examine temperatures during the thermosensitive period—when the sex of embryos is determined—and how this compares to the population’s thermal reaction norm, including the pivotal temperature that creates a 50/50 sex ratio. Extreme temperatures can lead to egg mortality, so we documented nest hatching success as well. This work was feature in the final chapter of my PhD dissertation, published in 2021.

Macroalgae blooms —
Global change has featured an increase in algae blooms. For instance, since 2011, Sargassum macroalgae has been proliferating in unprecedented quantities in the tropical Atlantic and collecting in coastal Caribbean nesting areas. Beached and nearshore Sargassum has ramifications for both nesting adults and emerging hatchlings. I have led several projects aimed at describing impacts, ranging from effects on nesting patterns (see this short paper, this conference paper, or this article) to egg incubation environments (see this article). The Sargassum issue appears here to stay! It is something we may have to learn to live with, and exploit where possible, rather than “fix.”

